The future of ophthalmic topical drug delivery is easy-to-use microdosing
EYE-GO is a MedTech company developing a game-changing delivery system for eye care treatment: MistGo®

MistGo® is a user-friendly, superior alternative to conventional eye drops
EYE-GO has developed a delivery system designed to help patients administer their eye medication in a more precise and user-friendly way

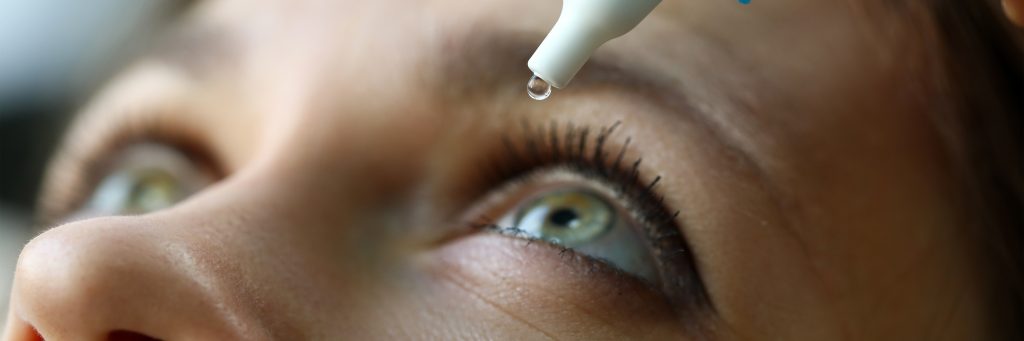
It is time to eliminate the flaws with dropper bottle
The way people take eye drops has hardly changed the last century.
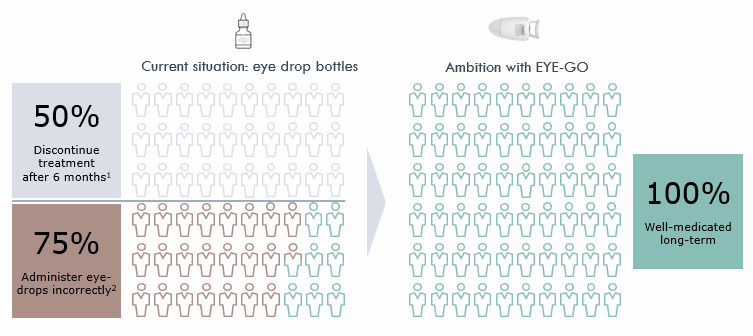
Eyedropper bottles are difficult to use and overmedicate the eye simply due to the size of a drop.
See EYE-GO’s one-pagers on micro-dosing and user test in the news section.
Only a fraction of patients achieves the full health outcome of treatment due to imperfections with current eye dop bottles
MistGo® helps patients stay well-medicated